Research Endeavor to Yield Hydrogen from Solar Power at TOBB ETÜ
9 YEAR(S) AGO
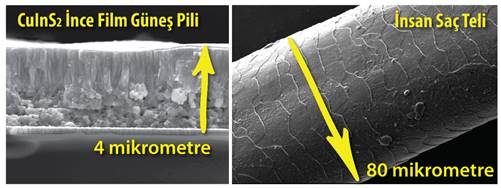
Thin film solar cells offer a great advantage over conventional type silicon based solar batteries when cost / power ratio is taken into consideration; therefore, it is a technology that is studied intensively in both academic and commercial dimensions. The main difference of this technology from the classical silicon based solar battery is that it is thinner as the name implies. The solar power, which can be absorbed at some 100 micrometer thickness with silicon solar cells, can be absorbed with the thin film technology at 1 micrometer thickness. Considering that the thickness one string of human hair is about 80 micrometers, thin film solar cells can be said to be 20 times thinner than human hair. As a natural consequence that, significant reductions in material consumption and production costs can be achieved.
Another advantage of the thin film technology is that the thin films can be produced on polymer and metal foil materials in addition to glass substrates, where by they can be used by way of integration to buildings and mobile vehicles. The thin film solar cells with copper-indium-sulfur (CSI) basis that is referred to as calchopyrite have been being studied intensively at the Energy Research Laboratory of TOBB ETÜ since 2009. At our laboratory, material consumption has been managed to be reduced up to 40 folds by way of spray-coating method, which could set an alternative to conventional methods of production. Through the method employed; we can spray-coat the aqueous solution at specific concentrations prepared with the starting chemicals that contain the elements of the thin film, which we intended to yield, on the preheated glass, metal, polymer or ceramic substrates. Therefore, we have reduced the production costs significantly relatively the costs of production of conventional solar cells.
Ultrasonic heads, which produce sound waves at such high frequencies that are beyond the hearing capacity of human ear, are used for the purpose of the spraying system. That allows for the solution submitted to be shrunk into droplets at micron sizes. At our laboratory, CIS based solar cells can be produced not only on glass substrates but also on polymer and metal foil in flexible form. The photovoltaics on flexible substrates by way of spraying pyrolysis method has first been produced by our research team at our laboratory. The solar cells' having a flexible form will pave the way for for numerous fields of application such as the use of the same as constructional components on buildings, the integration of the same to unmanned aerial vehicles and automobiles, and the use of the same as portable power generators.
At our laboratory, research and studies have also been being carried out on the photoelectrochemical solar cells, which enable artificial photosynthesis. As is known, plants are capable of transforming sunlight into chemical energy by way of photosynthesis. Using photoelectrochemical solar cells, the said capability of plants can be imitated and artificial photosynthesis can be performed. Those cells are the systems, which enable the transformation of solar energy into chemical energy, and enable and ensure uninterrupted electrical power supply at night or at times when lighting is poor. The photoelectrochemical solar cells is built simply by way of the placement of the photoactive semiconductive and counter electrode in the water, and the electrical energy generated by way of the radiation of the sunlight onto the semiconductive electrode causes the water to be electrolyzed, whereby enables the yielding hydrogen and oxygen gases. The hydrogen gas can either be used directly in such systems as fuel cells or be combined with carbon dioxide to yield methanol or organic hybrids. In this way, sources of energy suitable for use in a variety of fields can be yielded from the solar energy and the reduction of carbon footprint can be possible. Photoactive electrodes are produced through the research and study endeavors carried out at TOBB ETÜ's Energy Research Laboratory. Especially, the nano-structured zinc oxide films are, first, coated on glass by chemical bath storage method, and then are coated with CIS and/or indium sulfide nano-films through spraying method in order to improve the performance of those electrodes. Thus, high efficiency batteries can be produced.
Another subject, on which we carry out studies at our laboratory, is fuel cells. Fuel batteries convert the chemical energy of the fuel and oxidizer employed directly into electrical energy without combustion by way of an electrochemical reaction. As long as fuel supply to the system continues, they continue generating electricity. Among the fuel cells, the proton-permeable fuel cells, which are capable of operating at low temperatures (up to 120 ºC from the room temperature), are lightweight, small in size, easy and cost effective to design, are fairly promising to be used directly and as portable power supply for home applications and applications in the automotive industry. The proton-permeable fuel cells can be categorized into two groups, being direct methyl-alcohol fuel cells and hydrogen fuel cells, depending on the fuel used, and therefore, the amount of power to be generated thereby. Between the two categories; while the direct methyl-alcohol fuel cells are capable of generating a smaller amount of power and are relatively easier to design, the hydrogen fuel cells are capable of generating a larger amount of power. The most well-known advantages of fuel cells can be listed as high level of efficiency, low operating temperature, long service life, silent operation, easy design and size flexibility, broad range of fields of use and zero emission (in the case of hydrogen fuel cells).
The operating mechanism of a typical hydrogen fuel cell could be summarized in three main steps. Those steps are that the hydrogen gas is oxidized on the anode with the help of platinum and produces protons and electrons; the proton so produced is conducted to the cathode through the film and the electron so produced is made to move through the exterior circuit to be used for another useful purpose; and the combination of oxygen gas derived from the air or procured directly with the proton and electron and the production of water. Single cells are connected in series side by side in order to yield a larger amount of power (and also to increase the voltage generated), whereby a stack of fuel cells is formed. The most crucial component among the components of a fuel cell (1. Proton-permeable film (usually Nafion™); 2. Electrodes; 3. Fuel or oxidant diffusion layers (fabric or paper); 4. Double-sided plate (metal or composite); 5. Current collection plates (metal) 6. Terminal Plates (metal or composite)) is the proton-permeable film. Nafion™ ,Flemion™, Aciplex™ and Dow™ are the most known proton-permeable films. Among those films, the most industrially successful one is Nafion™ by Dupont. Therefore, Nafion™ is mentioned in a considerably large extent in the literature. However; Nafion ™ and similar materials also pose significant disadvantages. Among those disadvantages; the most critical ones are poor proton permeability under low humidity and high temperature, high methyl alcohol permeability, high water permeability, and observation of significant changes in the glass-transition temperature after water retention. Also, Nafion™ films are fairly expensive. Proton conductive films that will be alternative to Nafion™ and the other commercial films are produced at our laboratory. Those films are both display a higher performance than the other competitors and cost effective. Also, deliver canals for fuel cells are designed and fuel cell prototypes are produced at our laboratory. Those cells are suitable for use on automobiles, bicycles, golf carts, uninterrupted power supplies, aircrafts, underwater crafts, portable appliances and similar fields.